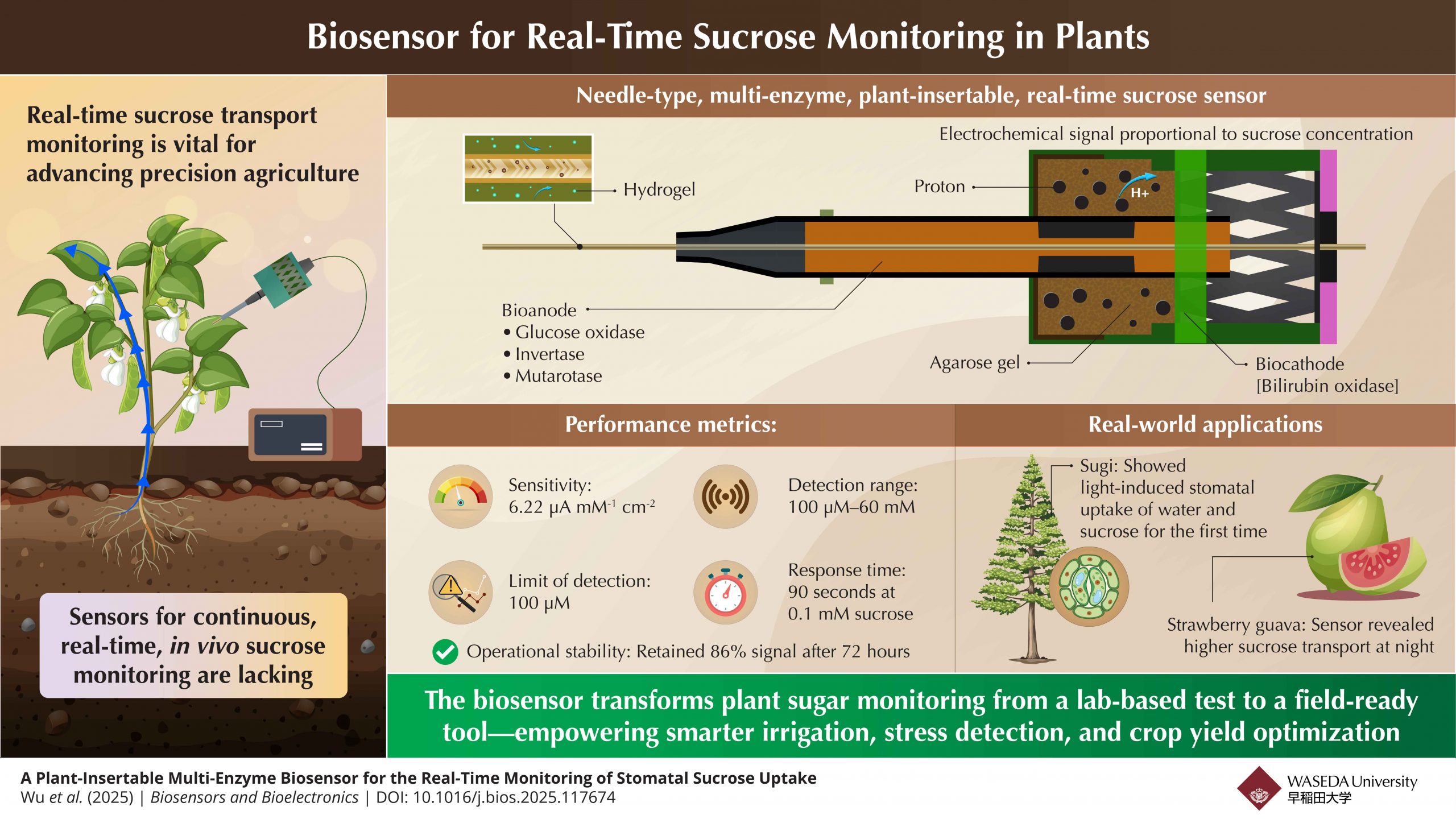
Researchers in Japan have developed a needle-type multi-enzyme biosensor that enables real-time monitoring of sucrose levels inside living plants. The sensor revealed daily patterns of sugar transport in strawberry guava and demonstrated that Japanese cedar can absorb sucrose through light-regulated stomata. The device, with its high sensitivity and stability, opens new avenues for studying plant physiology and optimizing agricultural practices through continuous, in vivo tracking of sugar dynamics under natural and controlled conditions.
Sucrose is a vital energy source in plants. It also drives growth and serves as an important signaling molecule during stress and development. Sucrose is a key product of photosynthesis and the primary form of sugar used for long-distance transport in plants. As such, its movement through plant tissues reveals much about its internal state. Yet, despite its importance, tracking sucrose in real time within living plants remains a persistent challenge.
One major challenge is the limited availability of in vivo sensors capable of capturing subtle physiological events, such as the movement of sucrose through plant tissues. Current techniques rely on destructive sampling or short-lived measurements. But sugars such as sucrose are dynamic—they shift depending on the time of day, light exposure, and plant developmental stage. So, the big question is whether these fluctuations can be measured continuously, in real time, especially to test emerging hypotheses about how plants absorb water and solutes through unexpected pathways like leaf stomata.
Now, researchers from Waseda University, led by Professor Takeo Miyake, and collaborating institutions have developed a flexible, needle-type enzymatic biosensor that can be inserted into plant tissues and report sucrose concentrations in real time. Miyake’s team at Waseda University included Shiqi Wu, Wakutaka Nakagawa, Dr. Saman Azhari, and Dr. Gábor Méhes. Professor Tomonori Kawano from the University of Kitakyushu and Yuta Nishina from Okayama University were also part of the team. Their findings were published in the journal Biosensors and Bioelectronics and made available online on June 8, 2025.
The biosensor integrates a multi-enzyme anode—glucose oxidase, invertase, and mutarotase—capable of catalyzing sucrose down to glucose and detecting its presence electrochemically. The cathode was bilirubin oxidase-based and worked with a unique agarose gel interface. The novel sensor achieved a high sensitivity, a detection limit of 100 µM, a detection range up to 60 mM, a response time of 90 seconds, and stable operation for over 72 hours. The device could be smoothly inserted into stems and fruits of plants with minimal damage.
“We designed the sensor specifically to capture sucrose uptake through stomata, which is a largely unexplored pathway,” says corresponding author Miyake. “The performance metrics were important, but what excites us most is the new biology it helped uncover.”
Using the sensor, the team observed a daily rhythm in sucrose transport within the stems of strawberry guava (Psidium cattleianum). Sucrose levels peaked during nighttime, consistent with the redistribution of photosynthetically generated sugars—a pattern that matches known growth cycles in many plants.
Further interesting findings came from experiments with the Japanese cedar (Cryptomeria japonica). Here, the researchers immersed cedar leaves in a sucrose solution and alternated between light and dark conditions. The biosensor, embedded in the plant’s stem, detected rising sucrose concentrations only during light exposure, when stomata are known to open. This suggested that sucrose could enter through the leaves and be transported internally, demonstrating stomatal-mediated sucrose uptake in a higher plant for the first time.
To confirm that water—and the dissolved sucrose—was indeed entering through stomata, the researchers used water labeled with the stable isotope oxygen-18. Analysis showed higher isotope levels in illuminated leaves, further supporting the hypothesis. They also observed a time lag of around 45 minutes between light exposure and sucrose increase in the stem, matching known transport speeds in plant phloem.
“This is the first time we’ve been able to directly track soluble sugar uptake through stomata in real-time,” said Miyake. “It challenges long-standing assumptions about how plants acquire water and nutrients.”
The authors note that the current version of the sensor is designed for short-term lab experiments, but future iterations may include wireless transmission and reduced invasiveness, making it suitable for long-term monitoring in the field. They also point out that the presence of secondary metabolites in plant sap did not interfere significantly with the sensor’s accuracy, due to the selective enzyme design.
In the future, the researchers want to apply the sensor for monitoring sugar dynamics in roots, seeds, and reproductive organs of plants. This could help create new strategies in crop yield optimization, stress detection, and growth modeling. As Miyake puts it, “If we can measure what the plant is doing in real-time, we can start to think about growing crops more intelligently.”